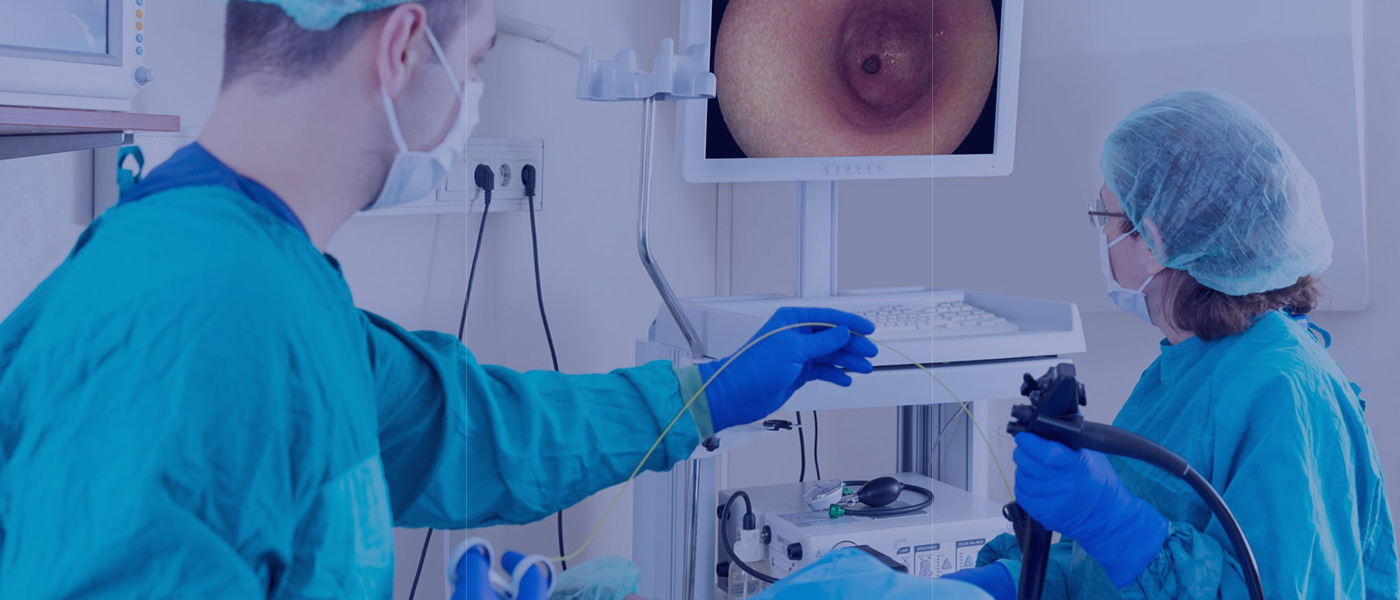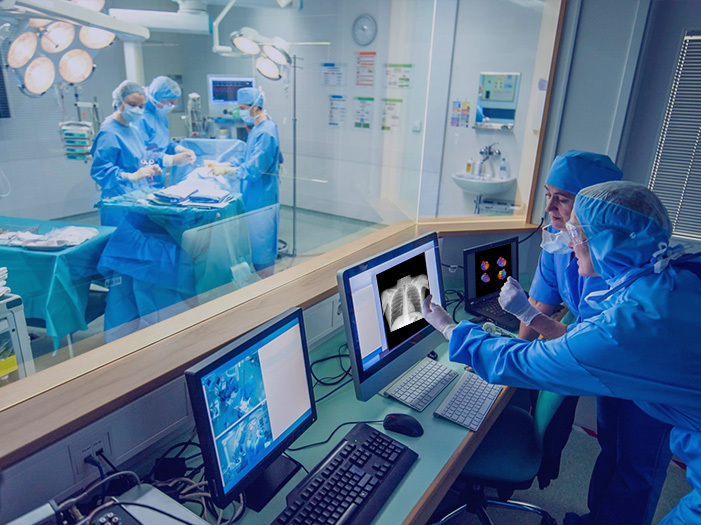
The Company has developed a series of innovative devices including those key ones to treat atrial fibrillation, resistant hypertension and bladder cancer, leading the cryotherapies of prevalent diseases in the world. The company's innovation also includes the non-cryo endoscopic clip for anastomosis. All these products have been recognized as “Innovative Medical Devices” by NMPA, and are protected by the patents worldwide.
Consistent focus on the integration of industry, science, and research, and continuously develop innovative products to meet clinical needs
近日,由宁波胜杰康生物科技有限公司自主研发的哮喘冷冻消融系统获得美国食品药品监督管理局(FDA)"突破性器械(Breakthrough Device)"认定,成为国际首个获此认定的哮喘冷冻消融产品。
2025年12月15日,宁波胜杰康生物科技有限公司自主研发的抗胃食管反流系统(包含“植入式抗胃食管反流器”和“食管测量工具”) 获得国家药品监督管理局(NMPA)批准上市(植入式抗胃食管反流器注册证编号:国械注准20253132592;食管测量工具注册证编号: 浙械注准20252021006)。